Medical Device Risk Assessment Training . Web this course will help you: Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Identify the key requirements of iso 14971:2019. Interpret and communicate the key requirements. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to.
from www.orielstat.com
Identify the key requirements of iso 14971:2019. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Interpret and communicate the key requirements. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Web this course will help you: Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at.
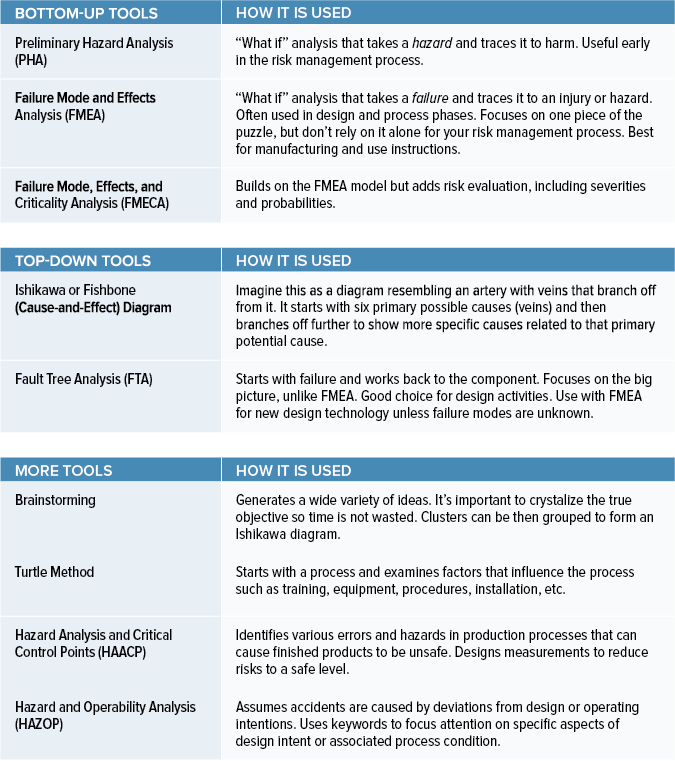
Choosing the right medical device risk management tools
Medical Device Risk Assessment Training Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web this course will help you: Interpret and communicate the key requirements. Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Identify the key requirements of iso 14971:2019.
From mungfali.com
Risk Assessment Template Medical Device Medical Device Risk Assessment Training Interpret and communicate the key requirements. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Identify the key requirements of. Medical Device Risk Assessment Training.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Assessment Training Web this course will help you: Identify the key requirements of iso 14971:2019. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web the first step is. Medical Device Risk Assessment Training.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Risk Assessment Training Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Interpret and communicate the key requirements. Web the first step is the development of a risk plan that. Medical Device Risk Assessment Training.
From mungfali.com
Risk Assessment Template Medical Device Medical Device Risk Assessment Training Web this course will help you: Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Interpret and communicate the key requirements. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks. Medical Device Risk Assessment Training.
From www.vrogue.co
5 Key Steps To Risk Assessments The Risk Assessment P vrogue.co Medical Device Risk Assessment Training Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Identify the key requirements of iso 14971:2019. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards. Medical Device Risk Assessment Training.
From www.ready.gov
Risk Assessment Ready.gov Medical Device Risk Assessment Training Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Identify the key requirements of iso 14971:2019. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Interpret and communicate the key requirements. Web iso 14971:2019 provides guidelines. Medical Device Risk Assessment Training.
From mungfali.com
Risk Assessment Logo Medical Device Risk Assessment Training Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,.. Medical Device Risk Assessment Training.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Assessment Training Interpret and communicate the key requirements. Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Identify the key requirements of iso 14971:2019. Web using case studies and interaction,. Medical Device Risk Assessment Training.
From academy.greenlight.guru
Introduction to Probabilistic Risk Assessment for Medical Devices Medical Device Risk Assessment Training Interpret and communicate the key requirements. Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web this course will help you: Web using case studies and interaction,. Medical Device Risk Assessment Training.
From www.americanpharmaceuticalreview.com
The Application of Risk Assessments for the Design and Development of Medical Device Risk Assessment Training Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Identify the key requirements of iso 14971:2019. Interpret and communicate the key requirements. Web this course will help. Medical Device Risk Assessment Training.
From www.slideserve.com
PPT Medical Device Risk Management Practical Overview & Challenges Medical Device Risk Assessment Training Identify the key requirements of iso 14971:2019. Interpret and communicate the key requirements. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web using case studies and interaction,. Medical Device Risk Assessment Training.
From www.meddeviceonline.com
Navigating The Universe Of Risk In Medical Device Development Medical Device Risk Assessment Training Interpret and communicate the key requirements. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web this course will help you: Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device,. Medical Device Risk Assessment Training.
From mungfali.com
ISO 45001 Risk Assessment Matrix Medical Device Risk Assessment Training Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Identify the key requirements of iso 14971:2019. Web this course will help you: Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web iso 14971:2019 provides guidelines. Medical Device Risk Assessment Training.
From blog.seerpharma.com
Application of ISO 14971 Risk Management to New Medical Devices Medical Device Risk Assessment Training Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web this course will help you: Web iso 14971:2019 provides guidelines. Medical Device Risk Assessment Training.
From www.pinterest.com
Risk Analysis Module Risk analysis, Analysis, Risk management Medical Device Risk Assessment Training Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Interpret and communicate the key requirements. Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Identify the key requirements of iso 14971:2019. Web the first step is the. Medical Device Risk Assessment Training.
From healthcaresecprivacy.blogspot.in
Healthcare Exchange Standards How to apply Risk Assessment to get your Medical Device Risk Assessment Training Web this course will help you: Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Identify the key requirements of iso 14971:2019. Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Web iso 14971:2019 provides. Medical Device Risk Assessment Training.
From medicaldevicehq.com
Performing medical device risk evaluation Medical Device HQ Medical Device Risk Assessment Training Web this course will help you: Web iso 14971:2019 provides guidelines for manufacturers to establish, document and maintain a risk management process to. Web the first step is the development of a risk plan that identifies the intended medical use of the device and addresses the risks at. Identify the key requirements of iso 14971:2019. Web the process described in. Medical Device Risk Assessment Training.
From www.greenlight.guru
Understanding ISO 14971 Medical Device Risk Management Medical Device Risk Assessment Training Web the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device, to. Web this course will help you: Web using case studies and interaction, you will practice identifying and analyzing potential product and process hazards, fmea,. Web the first step is the development of a risk plan that. Medical Device Risk Assessment Training.